 |
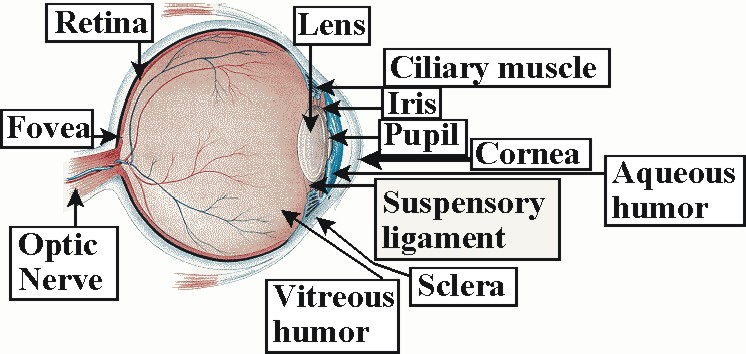
The Eye
This lab further reinforces your understanding of the the basic concepts in the images lectures. It also introduces a greater depth of knowledge about the visual system. After you carefully read this lab you can log on to Beachboard and take the short written test.
Introduction
As you know from class lecture, the visual system starts with the eyes and retina, travels to the lateral geniculate nucleus, to the primary visual cortex, and then to either the dorsal or ventral pathways. In this lab you will read about the eye and retina. You will learn the important cell types, the overall structure of the eye, and their informational and computational role.
The Eye and Retina
 |
The eye has two important functions; to gather and focus light reflected or projected from the visual field into an understandable image, and to convert the gathered image into electronic signals for processing. Starting in the front of the eye: The eye's hyaline, crystalline cupola, the cornea, measures only .5mm thick in the average adult. Nevertheless, it has five layers; epithelium, Bowman's membrane, stroma, Descemet's membrane and the endothelium. The cornea both to protects the eye and to supplies two-third's of the eye's refractive capabilities. Though the cornea lacks blood vessels, it surpasses all other bodily organs in its number of nerve endings. Where the cornea ends, the sclera (sometimes called the sclerotic or sclerotic coat) begins. The sclera is the tough white fibrous outer envelope of tissue covering the remainder of the eyeball.
In the space between the cornea and the iris, called the anterior chamber,
one finds a clear, watery fluid known as the aqueous humor. The
aqueous humor nourishes the cornea and the lens. Behind the anterior
chamber, dividing the front and back of the eye (posterior chamber), one finds
the iris. To the casual observer, the most striking features of the
iris are its center hole (pupil) and its color. Tiny pigment cells
called melanin provide the iris with a color, texture, and pattern as inimitable
as one's fingerprint. However, the iris' function, to control influx
of light through the pupil in a manner similar to the aperture of a camera, is
controlled by two sets of muscles. The sphincter muscle runs around the edge of the
pupil and contracts in bright light so as to constrict (narrow) the pupil. The dilator muscle runs radially through the
iris and dilates (widens) the pupil in response to tenebrous illumination.
Just behind the iris, and sharing the iris' blood supply, lies the ciliary
muscle or ciliary body. Fiberous tissue called zonules or suspensory
ligaments connect the ciliary body to the lens. These zonules suspended
the lens inside the eye. The lens is composed of crystallins.
Crystallins comprise a heterogeneous group of water-soluble structural proteins found in cells of the vertebrate
lens, and responsible for its refractory properties and transparancy. The
crystallins family contains four major groups; alpha, beta, gamma, and
delta. there are also several minor groups. Alpha, beta, and delta crystallins occur in avian and reptilian
lenses. All other lenses, including our own contain alpha, beta, and gamma crystallins.
The lens itself consists of the nucleus, the harder inner part of the lens, and
the cortex, a softer material surrounding the nucleus. Both nucleus and
cortex are encapsulated within a bag. The ciliary muscle has two functions.
First, it produces the aqueous humor that nourishes the cornea and lens as
described earlier. Second, the ciliary muscle together with its
zonules automatically adjust the focal length of the lens of the eye to permit retinal focus of images of objects at varying
distances within the visual scene. This process is normally referred to as accommodation.
Ciliary muscle contraction causes zonule relaxation, resulting in lens
thickening, and the corresponding increase in the eye's ability to focus upon
close objects. Ciliary muscle relaxation causes zonule contraction,
thinning the lens and increasing the eye's ability to focus upon distant
objects. Unfortunately, normal aging brings with it calcification of the ciliary body muscle and lens
causing presbyopia (farsightedness).
Behind the lens, in the chamber that comprises approximately two-thirds of the total volume of the eye, and often called the vitreous chamber, one finds the vitreous humor (also known as vitreous). The vitreous humor is a gelatinous, diaphanous substance consisting primarily of water and expanding the sclera like water in a balloon so as to give it shape and resisteance. The vitreous humor begins with viscosity comparable to egg white in children, but gradually thins with age. In some cases, the vitreous humor peels away from the back of the eye as it thins causing a condition prevalent in older populations called posterior vitreous detachment (PVD). Though "floaters," the perception of spots or web-like structures, or flashes of light often result from PVD, it usually is not considered serious unless the retina becomes torn or detaches from the back of the eye.
Finally, the retina is a multi-layered organ covering the surface of the vitreous chamber from the ciliary muscles to the optic nerve. In all there are nine layers in the retina proper and the Pigment Epithelium Layer (PE). Shown below are, from bottom to top, Pigment Epithelium Layer (PE), Layer Rods and Cones (R&CL), The Outer Limiting "Membrane" (OLM), The Outer Nuclear Layer (ONL), The Outer Plexiform Layer (OPL), The Inner Nuclear Layer (INL), The Inner Plexiform Layer (IPL), The Ganglion Cell Layer (GCL), The Nerve Fiber Layer (NFL), and the The Inner Limiting "Membrane" (ILM).
| Stained cross-section of the retina revealing its nine
layers.
Image courtesy of The Virigina-Maryland Regional college of Vetrinary Medicine |
For the purposes of this lab, the most obviously layer with which to begin is the ninth layer: Layer Rods and Cones (R&CL), also called the bacillary layer. The bacillary layer contains the photosensitive cells of the retina, the roods and cones. There are approximately 100 to 125 million rods and cones in the retina of each eye. Only about six million of these are cones. The vast majority of cones are concentrated in densely populated oval area of the Bacillary layer having a radius of about 3 by 5 mm called the fovea or macula retinae (literally, retinal stain). The fovea is located temporal (off center in the direction of the temple) to the optic nerve and at the rear of the eye. The fovea has both rods and cones, however, the central fovea consists exclusively of cones.
Rods
 |
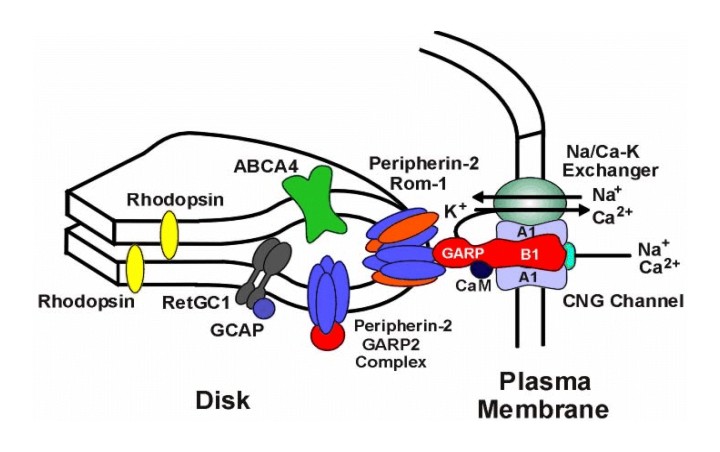
Rods constitute the vast majority of photosenstive cells in the retina. Unlike most cells, rods and cones are actually emitting their highest level of neurotransmitters into their synaptic clefts in total darkness. The effect of light is to decrease that neurotransmitter emission. To see how this works, let's start with some basic rod anatomy. The outer segment of the rod is a compartment of the cell that functions to capture light and initiate the conversion of light into electrical signals (i.e., phototransduction). Each out segment contains approximately 900 (mouse) to 2000 (frog) of discs surrounded by cytoplasm and encased in a plasma membrane. The discs themselves are composed of two flattened membranes folded over to form a rounded disc rim. Each disc contains molecules of rhodopsin. Rhodopsin (also called visual purple) is a redish thermolabile protein that is the photosensitive pigment in the rods of marine fish and the majority of higher vertebrates. There are approximately 108 molecules of rhodopsin in each rod, each is composed of opsin and retinal. Opsin refers to a class of colorless proteins that in combination with retinal or a related prosthetic (i.e., removable) group form a visual pigment. Retinal is a chromophore , but it's combination with opsin determines the exact light sensitivity spectrum of rods. That is, retinal is a member of chemical group capable of selective light absorption . Specifically, retinal is a yellowish to orange aldehyde C20H28O derived from the oxidative enzymatic splitting of absorbed dietary carotene (and has some properties of vitamin A) that in combination with proteins (opsin) forms the visual pigments of the retinal rods. |
|
|
|
Figure of Rod |
Figure of a disc outer rim with rhodopsin molecules and plasma membrane gate. Courtesy of Dr. Robert Molday |
Photons of light are absorbed by the retinal component of rhodopsin. When a rhodopsin molecule absorbs a photon of light, it rapidly bleaches, transforming isomerically to the all-trans-configuration, R* along with accompanying changes in the protein moiety. Photolyzed rhodopsin, R*, is unstable and the all-trans-retinal breaks away and becomes all-trans-retinol (i.e., vitamin A) which acts as a catalyst that binds to and activates hundreds of
|
molecules G-protein (transducin, T). Transducin is a G protein, meaning it is a member of a family of similar heterotrimeric (i.e., three different sub-units) proteins that compose the intracellular protein layer of the plasma membrane. G-proteins bind to activated receptor complexes where they, through conformational changes, cyclic binding, and/or hydrolysis of GTP, directly or indirectly alter membrane channel gating thereby effecting influx or efflux into a cell. Specifically, transducin has three subunits; β, γ, and α. The α subnuit is bound to a molecule of GDP (guanosine diphosphate). When activated by the all-trans-retinol, the tight bond between the α subnuit of the transducin and the GDP molecule is broken and the GDP is replaced with GTP (guanosine triphosphate). GTP-Tα separates from the β and γ subunits of the transducin molecule to form Transducin βγ (Tβγ ), which then binds to the inhibitory subunits, γ of phosphodiesterase (PDE) removing them from the PDE to create an active enzyme, cGMP-phosphodiesterase (PDE*). cGMP-phosphodiesterase stimulates hydrolysis of cyclic guanosine monophosphate (cGMP). That is, PDE* splits the cGMP into fragments by the addition of water, the hydroxyl (the univalent radical OH) group being incorporated in one fragment, and the hydrogen atom in the GMP. Each PDE* molecule can hydrolize 103cGMP molecules a second. To borrow from the Matrix, cGMP molecules "are the gate keepers." cGMP bonds directly to the cytoplasmic face of cGMP-gated channel at at least three points to activate the channel. Once the cGMP is broken down, the channel closes and the influx of positively charged Na+ is stopped. When enough Na+ are closed, the steady stream of -50pA ends and the rod then becomes hyperpolarized to about -70mv from a normal polarity of about -40mv. |
|
|
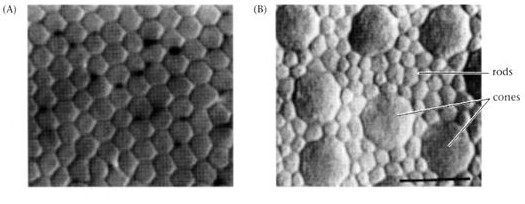
Scanning electron Micrograph of rods and 2 cones in the primate retina Image courtesy of thinkquest |
|
|
|
|
|
Animation of light transduction in rod
|
Once activated by a photon, a single rhodopsin molecule can lead to the breakdown of 105 cGMP molecules. The entire chemical cascade takes about 100msec.The hyperpolarization of the rod causes a decrease in the release of neurotransmitter (which is thought to be glutamate) to ganglion cells. The mechanisms are largely the same in the case of cones. In the next section I'll briefly discuss light adaptation and then we'll look at cones.
Rod and Cone Light Adaptation
When you walk out into the sunlight or from sunlight to a dark room, your vision is affected dramatically at first, and then slowly returns to more normal levels. The cellular mechanism that is responsible for this adaptation to the current light level is called light adaptation. Light adaptation allows the eye to operate over a much larger range of ambient light levels.
Historically, the first person to systematically investigate light and dark adaptation was a German physiologist Hermann Rudolph Aubert. Aubert studies in Berlin lead to an M.D. in 1850. In 1854, Aubert assumed the post of Privatdozent of physiology. Aubertís early publications include zoological investigations of insect thorax muscles. However, by 1854 Aubert was studying and publishing in human physiology exclusively, especially problems of psychophysics. In 1854 he published several articles on the conception of movement and orientation. 1865 saw Aubert become a full-professor of physiology. During this year he also conducted his investigation of dark adaptation (a phenomenon he named). Dark adaptation describes the eye's ability to regain its sensitivity in the dark following exposure to bright lights.
To study adaptation, one must first determine the light sensitivity of the eye, referred to as the absolute intensity threshold. The absolute intensity threshold is the minimum luminance capable of producing a visual sensation. With some simple equipment, anyone can measure the absolute intensity threshold. Go into a photography dark room or any room sealed from light, and allow your eyes to adjust for 5-10 minutes. Using a precise light source, raise the luminance until you can detect the light's presence. Aubert measured the absolute intensity threshold by measuring the amount of electric current necessary to cause the glow on a platinum wire to become visible. Among Aubert's results was his conclusion that the eye's sensitivity could increase approximately 35 times after prolonged exposure to darkness. Modern researchers have determined that a single photon can be detected by a rod.
Duplicity Theory is a theory about the relative roles of rods and
cones in normal vision. According to duplicity theory rods function to
facilitate night vision and peripheral vision. Rods have relatively poor
ability to discern detail, motion, and are insensitive to color. Cones
facilitate day vision, provide more detailed visual resolution, motion
detection, and color vision. Part of duplicity theory the fact that rods
and cones function exclusively within certain illumination ranges.
Depending upon the wavelengths of light available, at 0.034 cd/m2
(candles per cubic meter) rods begin to cease operating in vision as the light
increases and cones begin to operate in vision. The range of luminance
where both rods and cones are functioning to some degree is called the mesopic
range. Once luminance increases sufficiently beyond 0.034 cd/m2
so that cones exclusively mediate vision, and one reaches the photopic range.
When ambient lighting drops sufficiently below 0.034 cd/m2 rods
exclusively mediate photosensitivity and vision, and one reaches the scotopic
range.
 |
|
Figure courtesy of webvision |
Despite the length of time during which it has been studied, not everything is known about light adaptation. However, two mechanisms are fairly well understood. Calcium influx and efflux modulates light sensitivity in rods and cones by inhibiting the function of guanylyl cyclase. Guanylyl cyclase is the enzyme which takes the GTP from the light transduction process and synthesizes cGMP. Though I don't mention it in the discussion above, calcium in the form of Ca2+ also travels through the open the cation channels (cGMP-gated channels). In fact, approximately 1/7th of the ion flow through cation channels is Ca2+. During long exposure to higher levels of ambient light, the cation channels (cGMP-gated channels) close and the levels of Ca2+ slowly start to decrease. As the Ca2+ decreases, so does the calcium-mediated inhibition of guanylyl cyclase, and thus, the production of cGMP ioncreases. Calcium levels are also believed to expedite the inactivation of light pigments. As a result, more cGMP channels get opened and fewer visual pigments are available to absorb light. Together these two actions makes it more difficult for light to trigger the chemical cascade that causes rod and cone hyperpolarization. When one enters a dark room, the process just described works in reverse. The decrease in light increases levels increases the relative levels of Ca2+ , which causes increased inhibition of guanylyl cyclase and lowered production of cGMP. Lower levels of cGMP means fewer open gates, decreasing Ca2+ influx. Photosensitive pigments are slower to inactivate allowing for more active pigments to absorb photons.
Cones
 |
 |
|
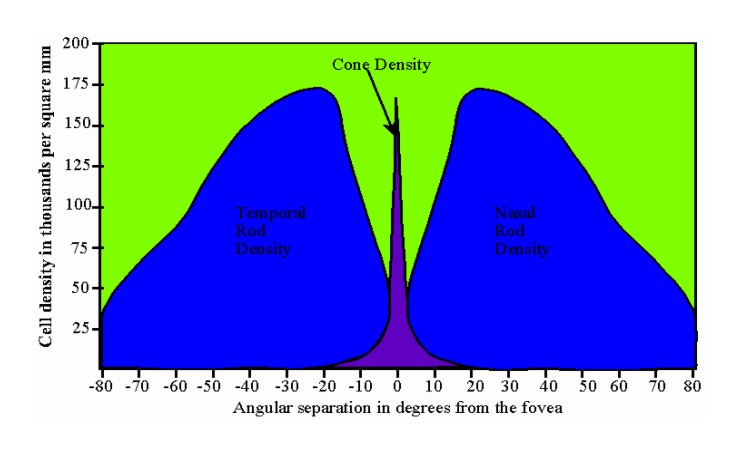
Distribution of Cones in fovea and periphery of retina Image courtesy of Ethan D. Montag |
|
|
Cones differ in a number of ways from rods. Firstly, rods outnumber cones approximately 100,000,000-120,000,000 to approximately 5,000,000-7,000,000. The relative distribution of rods and cones varies as well. Cone incidence increases dramatically from the fovea (all cones) to the periphery of the retina. The graph below illustrates the general distribution. |
|
 |
|
|
Figure of a cone |
Figure of rod and cone relative density in angular degrees from the fovea |
Like rods, the discs in cones contain 11-cis-retinal bound to an opsin. However, the molecule is often called iodopsin. Each cone pigment (often called red green, and blue) has a different version of opsin. Though only differing in several amino acid sequences, each opsin acts to fine tune the iodopsin molecule to a different wavelength sensitivity. Find below the various wavelength sensitivities for rods and cones given in light wavelengths.
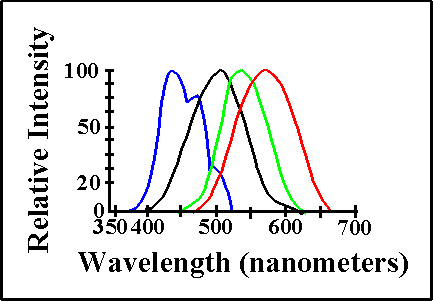 |
| Intensity of photoreceptor response to light wavelengths by rods, "red" cones, "green" cones, and "blue" cones. |
The Rest of the Retina
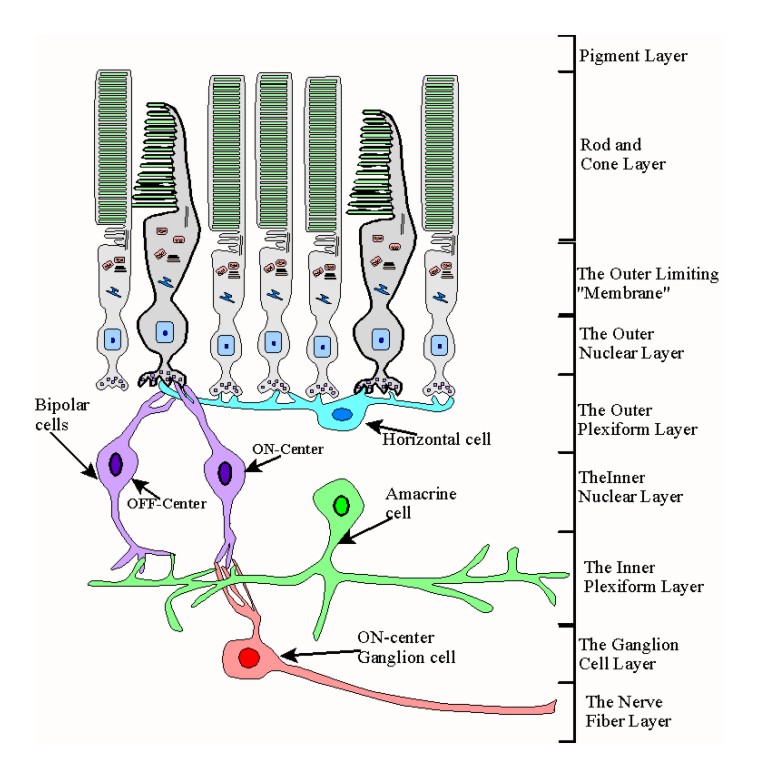 |
|
The remaining four retinal cell types and their relation to the nine histological levels discussed above |
http://faculty.washington.edu/palczews/articles/rhohetero.pdf
http://www.biochem.ubc.ca/Faculty/Molday4.jpg
http://www.eyedesignbook.com/
http://webvision.med.utah.edu/photo1.html&h=700&w=577&sz=166&tbnid=G-nUGPZrFXgJ:&tbnh=137&tbnw=113&start=3&prev=/images%3Fq%3Drods%2Band%2Bcones%26hl%3Den%26lr%3D%26sa%3DN
http://www.earthlife.net/mammals/vision.html
http://www.stlukeseye.com/anatomy/Aqueous.asp
http://hyperphysics.phy-astr.gsu.edu/hbase/vision/rodcone.html
http://education.vetmed.vt.edu/Curriculum/VM8054/EYE/RETINA.HTM